Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Kinetics of Reactions
Part c: The Collision Model of Reactions
Part a:
Reaction Rates
Part b:
Factors Affecting Reaction Rates
Part c: The Collision Model of Reactions
Part d:
Rate Equations
Part e:
Reaction Mechanisms
Collision Model
In Lesson 1b, we learned about four factors that affect the rate of a reaction - concentration, temperature, surface area, and the use of a catalyst. Increases in concentration most often lead to increases in reaction rate. Similarly, increases in temperature also increase reaction rates. Reaction rates are greater for situations in which the surface area of a solid reactant is greater. And finally, the use of a catalyst always increases reaction rates.
 In Lesson 1c, we will explain each of these cause-effect relationships using a particle model of reactions. The model is referred to as the Collision Model. The model presumes that reactant particles must collide with each other in order for a reaction to occur. Not every collision between reactant particles results in the breaking of bonds and the formation of a product. And so, the Collision Model makes a distinction between a collision and an effective collision. An effective collision is simply a collision between reactant particles that results in the formation of products.
In Lesson 1c, we will explain each of these cause-effect relationships using a particle model of reactions. The model is referred to as the Collision Model. The model presumes that reactant particles must collide with each other in order for a reaction to occur. Not every collision between reactant particles results in the breaking of bonds and the formation of a product. And so, the Collision Model makes a distinction between a collision and an effective collision. An effective collision is simply a collision between reactant particles that results in the formation of products.
An Effective Collision
According to the Collision Model, a collision between reactant molecules results in the breaking of bonds and the formation of product if:
- Colliding particles have a sufficient energy.
- Particles collide with the proper orientation relative to one another.
If both requirements are met - sufficient energy and proper orientation - then a collision between particles is considered an effective collision.


Consider several Legos bricks that have been snapped together to form a unit. You might think of them as analogous to atoms held together by bonds to form a molecule. Forces hold the Legos bricks together. To pull them apart takes some energy. In the same manner, pulling atoms apart in a molecule takes some energy.
Reactions involve breaking bonds among reactant molecules, the rearrangement of atoms, and the formation of new bonds in product molecules. Every reaction has a minimum energy, known as the
activation energy, that is required for the breaking of the bonds in reactants. This activation energy includes the energy required to break bonds and pull atoms apart. The Collision Model presumes that the energy required to pull atoms apart is supplied by the
kinetic energy of the colliding reactant particles. When particles collide, they have an amount of kinetic energy that is based upon their speed at the moment of impact. Not every reactant particle is traveling at the same speed. Some collisions involve the slower particles that have insufficient kinetic energy to meet the activation energy requirement. Other collisions involve higher speed particles with enough kinetic energy to meet the activation energy requirement and break bonds.

The
energy level diagram at the right depicts the potential energy level of reactants and products. The “hill” between reactants and products represents the minimum energy required to break bonds. The energy difference between the energy of the reactants and the
top of the hill represents the activation energy. If the activation energy for a reaction is 200 kJ/mol, then the colliding reactant particles must have at least 200 kJ/mol of kinetic energy to satisfy the activation energy requirement for an effective collision. If the particles do not satisfy this requirement, then they will bounce off each other without reacting and continue moving about the container.
The second requirement for an effective collision is that the colliding particles must have the proper spatial orientation. To illustrate this idea, let’s consider the following reaction:

The reaction can be thought of as a bromine ion replacing a chloride atom in the CH
3Cl molecule. The C-Cl bond must break and a C-Br bond must form. For this specific reaction, a poor spatial orientation results if the bromine ion approaches the CH
3Cl molecule from the chlorine atom’s side. The electron clouds of the relatively large chlorine atom will repel the bromine atom and prevent an effective collision. A more appropriate spatial orientation for this reaction would involve the bromine atom approaching the CH
3Cl molecule from the side opposite the chlorine atom. The smaller hydrogen atoms offer little repulsion of the bromine atom. This
backside attack meets the proper spatial orientation requirement for an effective collision.
This example is presented to illustrate what is meant by proper spatial orientation. The term refers to how a colliding particle approaches another reactant particle during a collision. It can refer to what part of the particles collide with each other and (at times) the angle at which they collide.
The Collision Model predicts that reaction rates are increased whenever a change is made that increases the frequency of effective collisions. Consideration must be given to meeting these two requirements. The more frequent and the more effective that collisions are, the greater that the reaction rate will be.
Explaining Concentration Effects
How does the Collision Model explain the effect of increasing reactant concentration upon reaction rate? The explanation hinges on the concept of collision frequency. When reactants are more concentrated within a reaction container, the reactants particles will collide more frequently. It is analogous to increasing the number of students in the lunch room. With a high concentration of students, collisions between students occur more frequently. With less students in the lunch room, there’s space to move about without colliding with a fellow student. If collision frequency increases, then a greater number of collisions will effectively lead to a reaction.
Explaining Temperature Effects
How does the Collision Model explain the effect of increasing temperature upon reaction rate?
Temperature is a measure of the amount of kinetic energy possessed by a sample. Higher temperatures correlate to a higher kinetic energy of particles. While changing temperatures will have no effect upon the required activation energy, it will affect the number of reactant particles that exceed this minimum energy requirement. With more particles exceeding the activation energy at higher temperatures, the likelihood that any given collision will be effective increases. This in turn increases the rate of a reaction.
We discussed the
temperature-kinetic energy relationship in Lesson 1b of the
Gases and Gas Laws chapter of this
Chemistry Tutorial. We mentioned that there is a distribution of speeds (and thus, of kinetic energies) in a sample of a gas. The distribution, often referred to as a
Maxwell-Boltzmann distribution, is often represented by a graph like the one shown below. The vertical axis of the graph represents the number of particles having a specific kinetic energy. At higher temperatures, there’s a broader range of kinetic energies than at lower temperatures. The high temperature curve on the graph is
flatter and broader because a higher proportion of the particles have higher kinetic energies. The activation energy is marked along the horizontal axis. The shaded areas represent the number of particles in the sample that exceed the activation energy. It is quite noticeable that this area is much greater for the higher temperature curve.
There is one more means by which increasing temperatures increase reaction rates. As temperatures increase, particles are moving about the container at higher speeds. This means that they will encounter collisions with other reactant particles more frequently. These more frequent collisions mean that there will be more effective collisions occurring every second. This results in an increase in reaction rate.
Explaining Surface Area Effects (for Solid Reactants)
We learned in
Lesson 1b that increasing the surface area of solid reactants increases the reaction rate. Solids are condensed states. Particles group up or aggregate together. Larger aggregates have a higher proportion of particles in the interior of the aggregate and a smaller proportion of particles on its exposed surfaces. Only particles on the surface of a solid aggregate are exposed to other reactant particles and able to participate in a collision. The interior particles are shielded from such collisions and will not be able to react.
Compare a 1-gram sugar cube to a 1-gram sample of granulated sugar. The sugar cube has a high proportion of unexposed sugar molecules in its interior. The sample of granulated sugar has a considerably smaller proportion of interior molecules shielded from other reactant particles. The sugar cube has a small surface area compared to the cumulative surface area of the many grains of sugar in the granulated form. Grinding a reactant into a fine powder is an even better method of increasing the surface area. This in turn increases the collision frequency and the reaction rate.
Explaining Catalyst Effects
 Catalysts
Catalysts speed up a reaction without actually participating as a reactant. They are never used up. They increase reaction rates by providing a different
pathway between reactants and products. The alternative pathway of a catalyzed reaction is one with a lower activation energy (
Ea). By providing a lower energy requirement, a catalyst makes it possible for a higher fraction of reactants particles to exceed the activation energy. This means there will be a higher frequency of effective collisions and an increase in the reaction rate.
The use of a Maxwell-Boltzmann distribution curve helps to explain how a catalyst increases the frequency of effective collisions and the reaction rate. The graph on the left represents the reaction without a catalyst. The graph on the right represents the same reaction run at the same temperature, but with a catalyst. Because there is no difference in temperature, the distribution curves are exactly the same. When using a catalyst, the activation energy (
Ea) is lower. Thus, a higher proportion of particles have a kinetic energy that exceeds this minimum threshold. The shaded portion represents those that satisfy the
sufficient energy requirement for an effective collision. It is noticeably greater for the catalyzed reaction.
Next Up
As we have seen many times in this Chemistry Tutorial, a particle model provides a reasonable, concept-based explanation for the patterns that we observe in the world of chemistry. In
the next part of Lesson 1, we will learn how Chemists perform experiments to determine quantitative information that predicts how a reaction rate depends upon concentration. But before you fast forward, slow down and take some time to internalize the ideas of Lesson 1c by putting them into practice. The
Before You Leave section provides several suggestions.
Before You Leave
- Try our Concept Builder titled Reaction Rates and the Collision Model. Any one of the three activities provides a great follow-up to Lessons 1b and 1c.
- Try our Science Reasoning Activity titled Reaction Rates. Any one of the four activities provides a great follow-up to Lessons 1b and 1c.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on The Collision Model. Save it to a safe location and use it as a review tool.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Identify the following statements as being TRUE or FALSE. If FALSE, identify what is incorrect about the statement or correct the statement.
- According to the Collision Model, every collision between reactant particles results in a reaction.
- The use of a catalyst will increase the reaction rate because catalysts increase the amount of kinetic energy that reactant particles possess.
- An increase in the temperature of a mixture of reactants will increase the kinetic energy of reactant particles.
- For a reaction to occur, reactant particles must be moving with a kinetic energy that is equal to or greater than the activation energy.
- Increasing the temperature of a mixture of reactants will increase the activation energy that is requied for the reaction to occur.
- Adding a catalyst can increase or decrease a reaction rate, depending on how much catalyst is added.
- An endothermic reaction has a positive activation energy and an exothermic reaction has a negative activation energy.
- It is possible for two colliding reactant particles to possess sufficient energy to break bonds but not undergo a reaction upon collision.
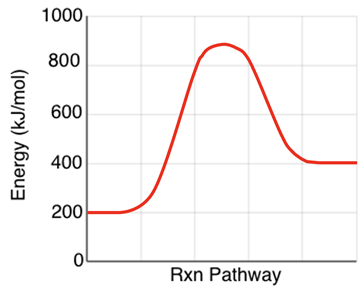
2. Consider the energy level diagram at the right for an endothermic reaction. On the diagram, label the reactants, the products, the ∆Energy, and the activation energy. Finally, identify the values of the ∆Energy and the activation energy.
3. Consider the reaction: H
2(g) + I
2(s) → 2 HI
(g). Identify four ways to increase the reaction rate for the reaction.
4. Grinding solid reactants into a fine powder will increase reaction rates. Which statement best describes the reason for this increase in reaction rate?
- Grinding reactants makes reactant particles more energetic resulting in more particles meeting the energy requirement.
- Grinding increases the activation energy for the reaction, resulting in more particles meeting the energy requirement.
- Grinding increases surface area and exposes a greater portion of the reactant particles to potential collisions.
- Grinding the solid results in a reaction pathway that has a lowered activation energy.
5. Reactions occur more rapidly at higher temperatures. Which
two statements best explain why increasing temperatures increase reaction rates? Pick two statements.
- Higher temperatures cause reactant particles to become more concentrated.
- Particle speeds increase, resulting in a higher collision frequency.
- The activation energy is lowered by increasing temperatures.
- Solid reactants assume larger grain size (less powdery) with greater surface area.
- A greater number of particles meet the energy requirement for an effective collision.